Last year the Craig Venter Institute announced that they had created an artificial bacterium. I thought I should follow up last months discussion of the Origin of Life with an overview of the claims, even though it is now considered 'old news' now.
Traditionally, genetically modified organisms (GMOs) are created by taking the DNA from an existing organism and identifying the gene responsible for a particular behaviour. In the most widely publicised cases, this has involved taking crop plants and adding genes from other species to add resistance to pesticides or to make the plant create an insect-repelling chemical.
DNA
DNA is a double-stranded molecule which means is consists of two polymers held alongside each other by Hydrogen Bonds, which are bonds between Hydrogen atoms and either Oxygen or Nitrogen atoms and are weaker than those which hold molecules together. The individual units of DNA are the four 'Bases': Adenine, Cytosine, Guanine and Thymine. The two strands of DNA line up with the bases paired off in a specific manner: a Cytosine always bonds with a Guanine and a Thymine always bonds with an Adenine.
A typical bacteria (for example e.coli) DNA contains around 4-5 million of these base pairs (bp) and contains around 4,000 genes. The function of most of these genes is known (or at least suspected), and like parts in a typical machine, some of these genes are essential for survival (such as ones which control essential cell functions) while others appear to be less important and may only be required under unusual circumstances (such as genes which allow bacteria to adapt to different food sources or which change behaviour when toxins are detected). Still more genes appear to serve no useful purpose or may be duplicates of existing genes.
A Less Than Minimal Genome
The goal of the artificial genome project was to create a minimal genome which contained only the essential genes. The e.coli genome is quite large but there are bacteria with much smaller genomes, such as Mycoplasma genitalium. This actually has the smallest genome of any non-symbiotic bacteria at only half a million base pairs and around 520 genes. This was the original target of the experiment and the researchers wanted to determine which of its genes were essential for growth under laboratory conditions. They made mutations where some of the genes had been disrupted but ignored genes where the function was already known to be essential. The bacteria has 480 genes which produce proteins and all but 100 of these appeared to be essential. The functions of some of the essential genes are still unknown.
Unfortunately, m.genitalium grows very slowly so the lessons learned from that were applied to other mycoplasma bacteria. The DNA they finally used was from m.mycoides which has a genome roughly twice the size.
Assembling the Genome
The new artificial genome would contain all of the essential genes without any of those considered to be unnecessary (or at least, not critical for survival in the well defined laboratory conditions). The sequence was split into shorter fragments of around 1000bp and each of these fragments was manufactured from individual bases. These shorter lengths were combined together in a series of steps to ultimately produce the full length DNA. This results in DNA which should contain sufficient genes for a viable cell, but which was made artificially instead of being assembled from fragments obtained from existing cells.
This new DNA also contained some 'watermark' regions to help identify the bacteria as containing the artificial DNA.
The Next Step
The only artificial part of the cell was the DNA, and that was build based on an existing genome, so the cell cannot really be described as truly artificial. It is still a major accomplishment to actually manufacture an entire chromosome since nothing on that scale had been achieved before.
All of the cellular machinery was already in place. Plain DNA on its own is useless and needs to be within a cell before it can perform any functions.
[This is the reason why viruses cannot function outside a cell and need to infect a host in order to replicate - a virus is typically composed of a strand of DNA (or the related RNA) enclosed within a protein shell. The shell is designed to both protect the contents and to help trick its way into a cell. Once inside, the cells DNA replicating machinery is co-opted to manufacture additional copies of the virus]
To make a truly artificial cell, there need to be structures in place to manufacture proteins from the templates present in the DNA (using processes called Transcription and Translation). If the cell is to be able to reproduce then there needs to be a method of duplicating the DNA and partitioning the new DNA and any important proteins and cellular machinery into two halves of the cell before it splits. All of this is complicated and not fully understood.
A truly artificial genome would also need to be written 'from scratch' instead of being assembled from pre-existing components. This is also beyond current abilities since we don't understand the functions of all the essential genes.
In summary, we are still a long way from being able to manufacture life from first principles. At the moment, nearly all of the stages currently require borrowing parts of existing cells. Back in the 18th century, it was generally believed that 'Organic' chemicals based around carbon chains could only be produced by living organisms, hence the name. At the moment we are still at that stage regarding living cells. The hurdles to artificial life are much greater than those to manufacturing chemicals but eventually they will be solved one at a time. It may take decades or even hundreds of years, but it is likely to be cracked eventually.
References
Essential genes of a minimal bacterium, Glass et.al, PNAS, 10 January 2006, vol. 103, no. 2, pp425-430
Creation of a Bacterial Cell Controlled by a Chemically Synthesized Genome, Gibson et.al, Science, vol. 329, 2 July 2010
In part 1, I discussed how early life may have arisen. In this part I will comment on one of the aspects of current terrestrial life - that of molecules symmetry or 'handedness'.
Molecular handedness, which chemists call Chirality, is a property of the 4 bonds which a carbon atom can form. If a particular carbon has 4 different groups of atoms attached to it, then there are two ways of attaching them such that one is a mirror image of the other. These are the two chiral forms, which are referred to as D- or L-isomers depending on the particular arrangement. In the 'skeleton' form of the amino acid Leucine, shown below, triangles mean the bond is pointing towards the viewer and the 'ladder' is a bond pointing away.
The centre carbon has the following 4 groups attached to it: Hydrogen NH2 'amine' group CO2H 'carboxylic acid' group CH2CH(CH3)2 branched carbon chain.
In terrestrial life, amino acids are almost exclusively the L-form and sugars are usually the D-form. There have been references to this in science fiction, such as the Arthur C. Clarke short story Technical Error where a scientist accidentally has his molecules changed to the opposite chirality and he is unable to metabolise any food.
If amino acid molecules are created by normal non-biological processes, there is usually a 50:50 mix of the D & L molecules (called a racemic mixture). Somehow, back when life was first beginning, there must have been a selective pressure on one form over the other.
Samples of amino acid have been retrieved from meteorite fragments and these turned out to have an excess of the L-isomer. Experiments have shown that polarized light can produce such a slight excess so conditions may have accidentally selected for a particular isomer and we are left with the legacy of those conditions.
Update (4/11/2011):
Earlier this week there was a discussion on the Simon Mayo show on Radio 2 which was about what would happen if the moon disappeared. This was prompted by a discussion of the film Despicable Me and the sequence where the moon was stolen.
It reminded me of a theory which stated that life wouldn't have developed on Earth if the moon didn't exist. The tides caused by the moon would have stirred up the precursor molecules and hastened any chemical reactions which would have led to life. An alternative version of this theory was presented in Star Trek: The Next Generation, where Q took Captain Picard back to the early Earth. Picard stirred up a 'soup' of organic molecules which mixed together to create life.
This week's science posting is in two parts. Part 1 covers a few theories about how life arose on Earth. Part 2 goes into a bit more detail on the molecules of life.
How Life came to Earth
There are two main theories on how life started on Earth: It came from Outer Space, and it started here on Earth. I will briefly describe the two approaches.
1. Panspermia
This theory claims that life came to Earth from elsewhere in the universe, and that there could be related organisms elsewhere in the galaxy. The approach was popularised by astronomers Fred Hoyle and Chandra Wickramasinghe, who believed that the process was still continuing today and that terrestrial outbreaks of disease could be caused by microbes brought to Earth by comets.
It certainly appears possible that life could be brought to Earth this way. Experiments have taken place where microbes have been taken into orbit and exposed to space where they have been exposed to cosmic rays. This took place in 2008-2010 when a piece of rock was fixed to the outside of the International Space Station. Space is a very harsh environment compared to the surface of the planet: there is no water or oxygen, and no ozone layer or atmosphere to protect against the harsh light from the sun. When the rock was brought back to Earth, laboratory tests showed that the bacteria had survived.
The surface of a comet is fairly hospitable by comparison. There might not be much of an atmosphere most of the time but there is some water and hydrocarbons which could provide a source of food. When the comet approaches the sun and warms up, clouds of gas billow out which could scatter any bacteria across the solar system where some could eventually arrive on Earth.
Evidence for Panspermia
Sadly there is no firm evidence for panspermia. No extra-terrestrial bacteria have been identified and there are no confirmed cases of bacteria of any kind being detected in comets. The closest we have is a meteorite which landed in Antarctica which has been identified as coming from Mars. This is the famous Allan Hills rock (known as ALH 84001) which had tiny features which looked like bacteria. Unfortunately these 'fossils' are much smaller than terrestrial bacteria so the identification as martian bacteria is not universally accepted.
Problems with Panspermia
While panspermia is theoretically possible, there is no evidence that it has taken place or that bacteria is present elsewhere in the solar system. The main problem is that it doesn't explain how life actually began, it only moves the problem to somewhere else, so even if panspermia is true, there is still the problem of how life began in the first place.
2. Terrestrial Abiogenesis
Abiogenesis is the process by which life arises from non-living matter. If life arose on Earth, rather than arriving from space, then the building blocks and conditions must have been present early in the Earth's history. The traditional view can be grossly over-simplified as: slowly over billions of years, different molecules reacted together to eventually create molecules capable of life.
This process might sound like a lottery - pick a molecule, see if it works, if it doesn't then try again. In reality the process is more likely to have been gradual: pick a molecule, if it doesn't work then modify it slightly and try again. This is more like the old Mastermind board game where you picked coloured pegs and tried to guess where your opponent placed them, or the Battleships game where you had to guess the locations of the boats. If a solution is 'almost correct' then it will be used as the basis for the next attempt, instead of throwing it away and starting again.
There have been attempts to replicate the conditions of early Earth to see if such molecules can spontaneously appear. The most famous of these is the 'Miller-Urey' experiment. Sparks were passed through an atmosphere of Water, Methane, Ammonia and Hydrogen to simulate lightning, which would break up the molecules to allow the atoms to re-join to form other molecules. The above gases were chosen because the elements they contain are sufficient to make 'amino acids' which are the building blocks for proteins and are vital for life (as we know it, at least).
After a week, simple sugars had formed, such as ribose (which has a chain of 5 carbon atoms, unlike the 6 carbons in glucose and fructose which I mentioned last week). The amino acid glycine (NH2CH2CO2H), had also formed.
Since then, molecules such as glycine and ethanol have been discovered in molecular clouds in space. Additionally the molecules adenine an glycine, which are known as 'Nucleotides' and are some of the building blocks of DNA, have been discovered in meteorites and comets. This proved that nature was capable of creating such molecules (and may suggest a compromise between panspermia and abiogenesis where the building blocks came from space but life arose on Earth).
The next step, from simple amino acids to actual life, is the part which causes the most problems. For life to form, we need a way of replicating molecules instead of waiting for them to spontaneously form one at a time. In cells there are two main types of molecular replication: manufacturing proteins from the template provided by the DNA (transcription/translation) and DNA replication itself (which occurs when cells divide).
Taking the first type of replication above, the first stage is Transcription. This takes a region of DNA which contains the 'recipe' for a protein and makes a copy of it, but as RNA instead of DNA (to put it simply, RNA is similar to DNA but uses slightly different building blocks). One current theory claims that early life might have been based on RNA instead of DNA so this stage can be ignored for now. I appreciate that I am skipping over a lot of detail here but some background reading can be found at here: Transcription Translation DNA Replication DNA Replication tutorial
At first glance it seems likely that the nucleotides which spontaneously formed may have joined together to form a primitive form of RNA (which stands for RiboNucleic Acid, which means it is formed from the sugar Ribose and Nucleotides, both of which have been identified in meteorites), but chemists have worked out the steps required and claimed that it isn't possible. An alternative chemical route has been suggested (Powner et.al, Nature, 14th May 2009) where each of the steps is plausible and all the of ingredients would have been available in the early environment.
Creating proteins from RNA uses a giant collection of molecules called the Ribosome, which is so complex and specialized that it cannot have spontaneously developed in its current form. There are suggestions that a much simpler 'proto-ribosome' formed, which was a much less complex enzyme which could have self-assembled (Agmon et.al, Nature Proceedings, March 2009).
In this way, life arose from simple molecules which were able to form in the 'primordial soup' which gradually led to more complex associations of molecules which ultimately led to something which we could identify as Life.
Further reading can be found here.
After last week's post about Yeast, I received a couple of questions asking for a bit of clarification. I thought it would be a good idea to post the questions and my response here.
Question 1
In the article you wrote:
'which is the classic bakers or brewers yeast'
With that statement, it took it to mean that they are one and the same yeast but having read further it would seem not to be the case. Have I read it properly?
Answer
Sorry if I made it a bit confusing. The traditional general purpose brewers yeast is indeed the same as bakers yeast. The other 2 yeasts I mention are a bit more specialist.
Question 2
Thanks Mike. I wonder how they isolate specific yeasts, as in the pombe one?
Answer
One way is to put a dilute solution of growing culture on an agar plate, which is a kind of jelly made using the growth medium, and leave the yeast to grow for a few days. After that you pick out individual colonies, which will usually have grown from single yeast cells. There is likely to be 100s of colonies of various types depending on what is present.
You can then do whatever tests are required to identify them, which these days will often involve some kind of genetic test. If you are wanting to brew wine or beer you can make trial runs with the different colonies to see how they affect the flavour. This is probably how they ended up with the different wine yeasts where you can buy yeast 'optimized' for different types of red or white wine.
This is the first in a short series of science-related posts where I will be explaining a bit of science behind some things which are of particular interest or relevence to me. I will be starting with fermentation since it is important in both baking and wine- and beer-making.
Types of Yeast
I apologise in advance for the use of the scientific names for the yeast but I need to be precise about which types of yeast I am talking about.
There are over 1,500 different types of yeast but the one which most people will be familiar with is called Saccharomyces cerevisiae, which is the classic bakers or brewers yeast. This has been used for centuries for this purpose. This is known as a 'top fermenting' yeast since, when making beer or wine, the live yeast forms a thick layer on top of the liquid.
Another yeast used in fermentation is Saccharomyces pastorianus (sometimes called S. carlsbergensis). This is a hybrid between S. cerevisiae and a naturally occurring yeast, S. eubayanus, which has been recently isolated from populations in South America.
A third type of yeast is called Schizosaccharomyces pombe. It gets its name from the Swahili word for beer and was isolated from strains used in East Africa to brew beer from millet. Along with S. cerevisiae it is used in research as a 'model organism' to investigate things such as signalling or communication between cells.
Yeast Metabolism
Yeast are typically most efficient when using simple sugars as a food. The common everyday sugar (known as sucrose) is a 'disaccharide' which means it is composed of two simpler sugar molecules (called monosaccharides) joined together.
In the presence of oxygen, the yeast grow and multiply rapidly which leads to the rapidly forming froth on top of fermenting liquids. After the oxygen has been used up, the yeast can then get on with their primary job which is fermentation:
Glucose → 2 molecules of ethanol and 2 molecules of Carbon dioxide. In brewing it is the ethanol we want, whereas in baking it is the carbon dioxide which is needed to make bread rise (Of course if you are making champagne, cider or any other 'fizzy' drink, you also want some of the carbon dioxide too).
Another way of writing this is:
C6H12O6 → 2C2H6O + 2 CO2
Brewing yeast can normally only directly consume the simpler monosaccharides so sucrose needs to be broken down in a process called 'Inverting' (this is what the 'Inverted Sugar Syrup' means on ingredient lists on manufactured foods).
Sucrose + Water → Glucose + Fructose
The above reaction is known as hydrolysis because it involves adding water to chemically change something. It can be done by boiling a sugar syrup with citric acid, which is a technique sometimes used to speed up brewing (at least in some homebrew circles).
As a chemical reaction, this can be written as:
C12H22O11 + H2O → C6H12O6 + C6H12O6
The two C6H12O6 on the right hand side look the same but they are actually Glucose and Fructose. They contain the same atoms but arranged differently.
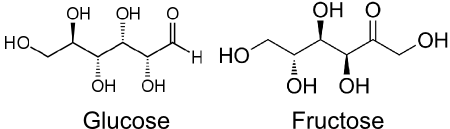
(Based on images from Wikipedia)
The yeast do not actually do this themselves but they produce an enzyme called Invertase which they release into their surroundings, where this reaction actually takes place.
Coming Soon
Future posts will cover such subjects as Astronomy, Chemistry and Biology in various combinations. Feel free to leave any comments, including requests for future articles.