This is the first in a short series of science-related posts where I will be explaining a bit of science behind some things which are of particular interest or relevence to me. I will be starting with fermentation since it is important in both baking and wine- and beer-making.
Types of Yeast
I apologise in advance for the use of the scientific names for the yeast but I need to be precise about which types of yeast I am talking about.
There are over 1,500 different types of yeast but the one which most people will be familiar with is called Saccharomyces cerevisiae, which is the classic bakers or brewers yeast. This has been used for centuries for this purpose. This is known as a 'top fermenting' yeast since, when making beer or wine, the live yeast forms a thick layer on top of the liquid.
Another yeast used in fermentation is Saccharomyces pastorianus (sometimes called S. carlsbergensis). This is a hybrid between S. cerevisiae and a naturally occurring yeast, S. eubayanus, which has been recently isolated from populations in South America.
A third type of yeast is called Schizosaccharomyces pombe. It gets its name from the Swahili word for beer and was isolated from strains used in East Africa to brew beer from millet. Along with S. cerevisiae it is used in research as a 'model organism' to investigate things such as signalling or communication between cells.
Yeast Metabolism
Yeast are typically most efficient when using simple sugars as a food. The common everyday sugar (known as sucrose) is a 'disaccharide' which means it is composed of two simpler sugar molecules (called monosaccharides) joined together.
In the presence of oxygen, the yeast grow and multiply rapidly which leads to the rapidly forming froth on top of fermenting liquids. After the oxygen has been used up, the yeast can then get on with their primary job which is fermentation:
Glucose → 2 molecules of ethanol and 2 molecules of Carbon dioxide. In brewing it is the ethanol we want, whereas in baking it is the carbon dioxide which is needed to make bread rise (Of course if you are making champagne, cider or any other 'fizzy' drink, you also want some of the carbon dioxide too).
Another way of writing this is:
C6H12O6 → 2C2H6O + 2 CO2
Brewing yeast can normally only directly consume the simpler monosaccharides so sucrose needs to be broken down in a process called 'Inverting' (this is what the 'Inverted Sugar Syrup' means on ingredient lists on manufactured foods).
Sucrose + Water → Glucose + Fructose
The above reaction is known as hydrolysis because it involves adding water to chemically change something. It can be done by boiling a sugar syrup with citric acid, which is a technique sometimes used to speed up brewing (at least in some homebrew circles).
As a chemical reaction, this can be written as:
C12H22O11 + H2O → C6H12O6 + C6H12O6
The two C6H12O6 on the right hand side look the same but they are actually Glucose and Fructose. They contain the same atoms but arranged differently.
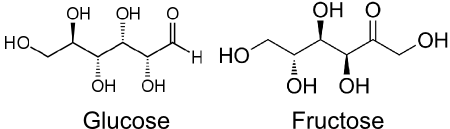
(Based on images from Wikipedia)
The yeast do not actually do this themselves but they produce an enzyme called Invertase which they release into their surroundings, where this reaction actually takes place.
Coming Soon
Future posts will cover such subjects as Astronomy, Chemistry and Biology in various combinations. Feel free to leave any comments, including requests for future articles.